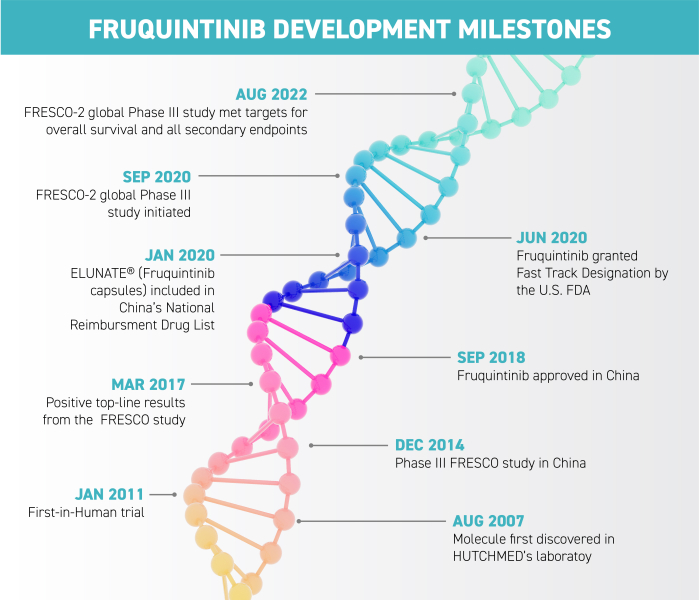
Chi-Med Announces Fruquintinib Granted U.S. FDA Fast Track Designation for Metastatic Colorectal Cancer-CliniExpert

Fruquintinib Plus Toripalimab Is an Effective Third-Line Treatment of Advanced Colorectal Cancer | Docwire News
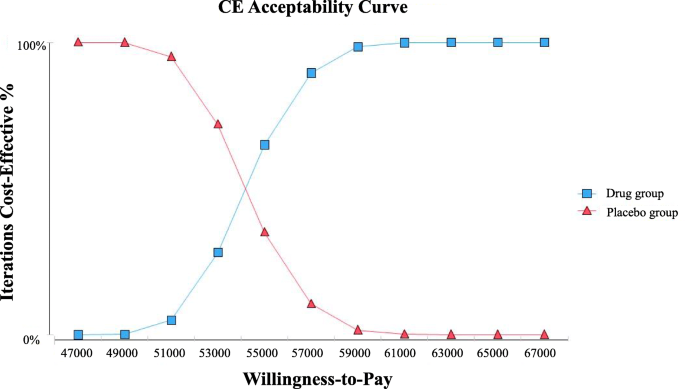
Cost-effectiveness analysis of fruquintinib for metastatic colorectal cancer third-line treatment in China | BMC Cancer | Full Text

HUTCHMED Announces that Takeda Receives U.S. FDA Approval of FRUZAQLA™ ( fruquintinib) for Previously Treated Metastatic Colorectal Cancer

StudyPages - Clinical trial with Fruquintinib in patients with Metastatic Colorectal Cancer who has not responded to previous treatment

Takeda Receives U.S. FDA Approval of FRUZAQLA™ (fruquintinib) for Previously Treated Metastatic Colorectal Cancer | Business Wire